aluminum valence electrons|Determine valence electrons using the periodic table : Bacolod There are two ways to find the number of valence electrons in Aluminum (Al). The first is to use the Periodic Table to figure out how many electrons Aluminum .
Boku to Misaki-sensei | ボクとみさき先生 [1080p - Softsub ] (French subs)
aluminum valence electrons,Mar 23, 2023
Find the valences of the chemical elements in a table and learn how they are .
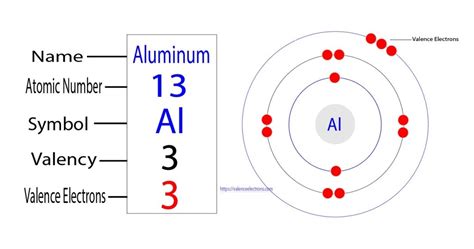
Aluminum (Al) is located in Group IIIA (Group 13), so it has 3 valence electrons. ️ Exercise \(\PageIndex{1}\) Tell which group of elements (alkali metals, .

Learn how to find the valence electrons of aluminum (Al) by following three steps: determining the total number of electrons, arranging them in shells, and ide.

Learn how to find the valence electrons of aluminum (Al) by following three steps: determining the total number of electrons, arranging them in shells, and ide. Write the configuration of Aluminum, assuming that it has lost its valence electrons. What happens to aluminum when it reacts with chlorine? Balance these . There are two ways to find the number of valence electrons in Aluminum (Al). The first is to use the Periodic Table to figure out how many electrons Aluminum .Learn how valence electrons determine the bonding patterns and reactivity of elements. Find out how to count valence electrons and see examples for aluminum and . An aluminum atom has three valence electrons. Do you think it will lose three electrons or gain five electrons to obtain an octet in its outermost electron shell? . Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2 s subshell and four in the 2 p subshell. We can write the configuration of oxygen's valence electrons as 2 .Learn how to determine the number of valence electrons for an element using the periodic table. See patterns, examples, and diagrams for main group elements, and . Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .The underlying core under aluminium's valence shell is that of the preceding noble gas, whereas those of its heavier congeners gallium, . Hence, the inner electrons of aluminium shield the valence . The electron configuration of aluminum is [ Ne] 3s 2 3p 1. In the above electron configuration, the highest energy level (3) is marked with green color. The 3 rd energy level contains 3s and 3p subshells. .aluminum valence electronsFigure 2.4.2 2.4. 2: Electron diagram for magnesium. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the n = 3 n = 3 energy level which is the highest occupied energy level for magnesium. This corresponds to the 2+ 2 + charge formed when magnesium forms an ion.
Determine valence electrons using the periodic table Figure 2.4.2 2.4. 2: Electron diagram for magnesium. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the n = 3 n = 3 energy level which is the highest occupied energy level for magnesium. This corresponds to the 2+ 2 + charge formed when magnesium forms an ion.
This periodic table shows the valences of element groups. The transition metals make use of the d-subshell, which can accommodate 10 electrons.The f-subshell holds 14 electrons and the g-subshell contains up to 18 electrons.Metals in the middle of the periodic table become more stable by emptying a shell, half-filling it, or completely .In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can . Aluminum is the 13th element of the periodic table so its atomic number is 13. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, an aluminum atom has thirteen protons and thirteen electrons. The number of neutrons in an atom can be determined by the difference between the .Jul 15, 2017. The number of valance electrons is 3. Explanation: The electron configuration for Aluminum is. 1s22s22p63s23p1. The first 10 electrons are in the filled first and second shells. This leaves only three electrons in the third shell 3s23p1. 13 −10 = 3. so there are only 3 valance electrons.
aluminum valence electrons|Determine valence electrons using the periodic table
PH0 · Valences of the Chemical Elements
PH1 · Valence electrons (video)
PH2 · Valence Electrons Chart for All Elements
PH3 · Valence Electrons
PH4 · How to Find the Valence Electrons for Aluminum (Al)?
PH5 · How to Find Valence Electrons for Aluminum (Al)
PH6 · How Many Valence Electrons Does Aluminum (Al) Have?
PH7 · Determine valence electrons using the periodic table
PH8 · Chemistry of Aluminum (Z=13)
PH9 · 3.1: Valence Electrons
PH10 · 10.6: Valence Electrons